Non-surgical cancer treatments include various minimally invasive techniques that destroy tumors without open surgery. They provide organ preservation, faster recovery, and fewer complications.
Ablation methods such as radiofrequency, microwave, cryoablation, and irreversible electroporation directly target tumors with energy-based technologies. They are widely used in liver, kidney, lung, and bone cancers.
In addition to ablation, treatments like embolization and high-intensity focused ultrasound (HIFU) offer effective alternatives for patients unsuitable for surgical resection. These methods are image-guided and repeatable.
Non-surgical options are especially valuable for high-risk patients. They provide effective local control, preserve quality of life, and can be combined with systemic therapies for enhanced outcomes.

Prof. Dr. Özgür KILIÇKESMEZ
Interventional Radiology / Interventional Neuroradiology
Prof. Dr. Kılıçkesmez holds the Turkish Radiology Competency Certificate, the Turkish Interventional Radiology Competency Certificate, Stroke Treatment Certification, and the European Board of Interventional Radiology (EBIR). In his academic career, he won the Siemens Radiology First Prize in 2008.
Why Is Non-Surgical Cancer Treatment Important?
Non-surgical cancer treatments are a significant advantage for patients who are not suitable for surgery for various reasons or who need supportive therapy alongside surgery. You can think of this as targeting weeds in your garden with a focused solution rather than removing each by hand. By focusing only on the problematic area, healthy tissues are spared as much as possible and patients can return to their daily lives quickly.
- After surgery, patients often need to stay in the hospital for an extended period. In contrast, with minimally invasive methods like interventional radiology, discharge on the same or next day is usually possible.
- Because no large incisions are made, tissue damage and pain are generally less.
- Some non-surgical methods can be repeated or applied to different sites if needed, allowing for flexible and tailored treatment as the disease progresses.
However, the choice of treatment always depends on the tumor type, stage, patient’s overall condition, and other relevant factors.
How Are Interventional Radiological Treatments Performed?
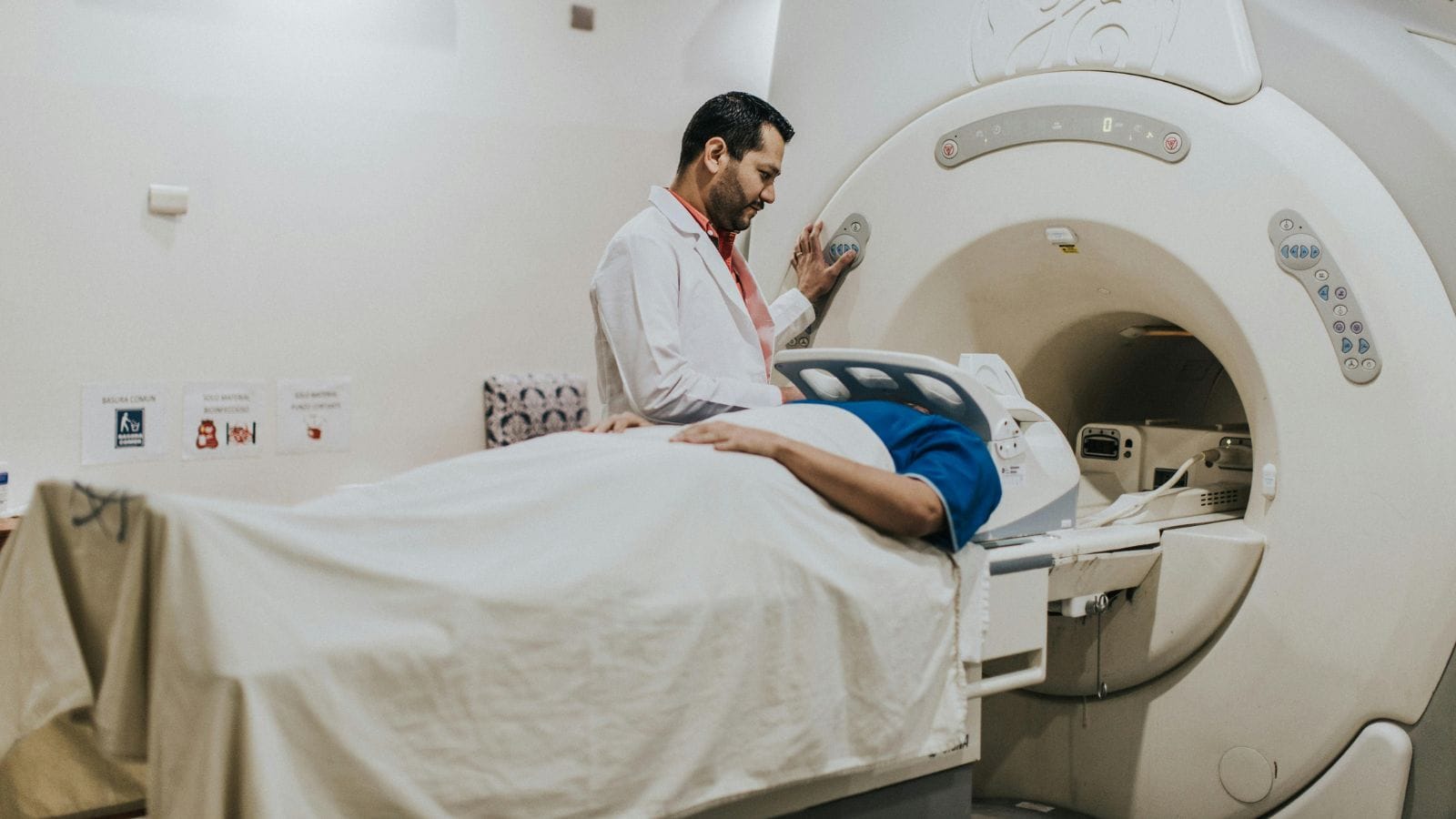
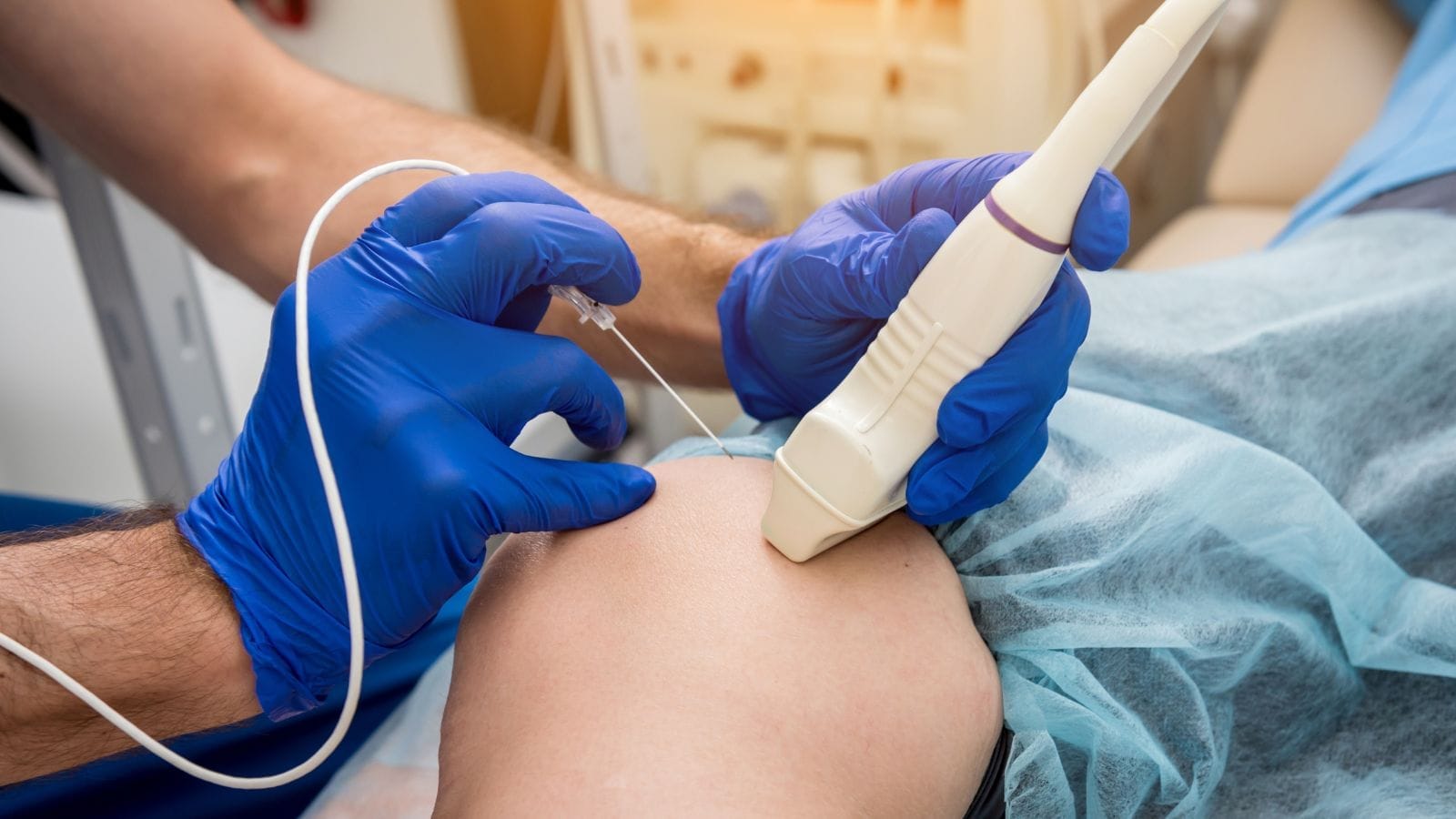
Interventional radiology covers highly precise, often “targeted” treatment techniques that are gaining increasing importance in cancer care. These methods typically use imaging technologies like ultrasound, computed tomography (CT), or magnetic resonance imaging (MRI) to guide thin needles or catheters to the tumor or its supplying vessels. Then, with the help of heat, cold, or special drugs, the target tissue is destroyed or significantly reduced.
How does the process work?
- First, detailed imaging is performed to determine the tumor’s size, location, and relation to nearby tissues.
- The patient is taken to the interventional radiology suite, and most procedures are done under local anesthesia or light sedation.
- Guided by imaging, the needle or catheter is advanced precisely to the tumor.
- The selected treatment is performed: heat, freezing, or administration of special agents.
The goal is much like a skilled marksman aiming for the bullseye—to destroy the tumor exactly at its location.
What Interventional Treatment Methods Exist?
Interventional radiology offers a wide array of techniques.
Radiofrequency Ablation (RFA)
RFA uses high-frequency radio waves to “burn” unwanted tissue or tumors. Think of a toaster—the resistors heat up and toast the bread. In RFA, a needle-like probe generates heat with electric current, destroying cancer cells.
Advantages: Particularly effective for small tumors in organs like the liver, lung, and kidney. Short hospital stay and relatively low complication rates.
Considerations: Success may vary for larger tumors or those close to major vessels.
Microwave Ablation (MWA)
MWA also uses heat to destroy cancer cells, but through microwave energy, providing faster and broader heating for some larger or more challenging tumors.
Advantages: Heat spreads more widely; suitable for some bigger or differently located tumors.
Limitations: Results depend on the tumor’s position, organ structure, and size.
Cryoablation
Watch a short video here.
Instead of “burning,” this method “freezes” the tumor. Extreme cold is achieved using argon gas, freezing and destroying the tumor. The frozen tissue can be seen as an “ice ball” on imaging.
Advantages: The extent of freezing is clearly visible on imaging, allowing for precise control.
Possible Downsides: Success still depends on the tumor’s location and size.
Irreversible Electroporation (IRE)
IRE uses short, high-voltage electrical pulses to create permanent pores in cancer cell membranes, leading to cell death—like making small holes in a balloon so it loses air.
Unique Feature: It does not use heat, so nearby blood vessels and bile ducts are less likely to be damaged.
Limitations: Requires special equipment; effectiveness depends on the tumor’s size and shape.
TACE (Transarterial Chemoembolization)
Commonly used for liver tumors. The goal is to block the tumor’s blood supply and deliver chemotherapy directly to it through a catheter, causing the tumor to be starved of oxygen and hit with high-dose chemo.
Advantages: Chemotherapy goes directly to the tumor, minimizing systemic side effects.
Applications: Especially for certain liver cancers or metastatic tumors in the liver.
TARE (Transarterial Radioembolization)
Watch a short video here.
Similar to TACE but uses radioactive particles (often Yttrium-90 microspheres) instead of chemotherapy. The radioactive beads provide localized internal radiation, killing cancer cells.
Difference: Focuses on radiotherapy with less collateral damage to surrounding tissues.
Advantage: Delivers localized radiation with minimal harm to nearby healthy tissue.
What Other Non-Surgical Treatments Exist?
Outside of interventional radiology, many other non-surgical cancer treatments are available, including:
Chemotherapy: Drugs delivered intravenously or orally to destroy cancer cells throughout the body.
Radiation Therapy: Uses high-energy rays to damage the DNA of cancer cells.
Immunotherapy: Boosts the immune system’s ability to recognize and destroy cancer cells.
Targeted Therapies: Drugs that target specific genetic or protein changes in cancer cells.
Hormone Therapy: Used especially for hormone-sensitive cancers (e.g., breast, prostate) to block or lower hormone effects.
For Which Cancer Types Are These Interventional Treatments Used?
Interventional radiology techniques are often used for early-stage or small tumors in the liver, lung, kidney, and some bone cancers. Even in advanced or metastatic cases, they can help reduce tumor burden, relieve pain, and improve quality of life.
For example:
- Liver cancers (hepatocellular carcinoma, metastatic lesions)
- Lung nodules (small, accessible ones)
- Kidney tumors (especially small ones)
- Bone metastases (for pain relief or to reduce fracture risk)
The applications are expanding, and multiple techniques can be combined and customized for each patient.
What Preparation Is Needed for Interventional Treatments?
Once a decision is made for these procedures, patients undergo comprehensive evaluation—like preparing your car for a long journey. Blood tests, heart and lung function, and other health conditions are reviewed. Consultations with nuclear medicine, oncology, pulmonology, or cardiology may be required.
Before the procedure:
- Usually, a few hours of fasting is requested.
- Medications are reviewed. Adjustments to blood thinners or diabetes drugs may be necessary.
- The type of anesthesia (local, sedation, or general) is determined.
Each treatment has its own steps, but the main goal is to keep the patient as safe and comfortable as possible.
What Happens During and After the Procedure?
Most interventional radiology procedures are performed in specialized, sterile rooms similar to an operating theater. The patient lies on the table, usually connected to monitors. During the procedure:
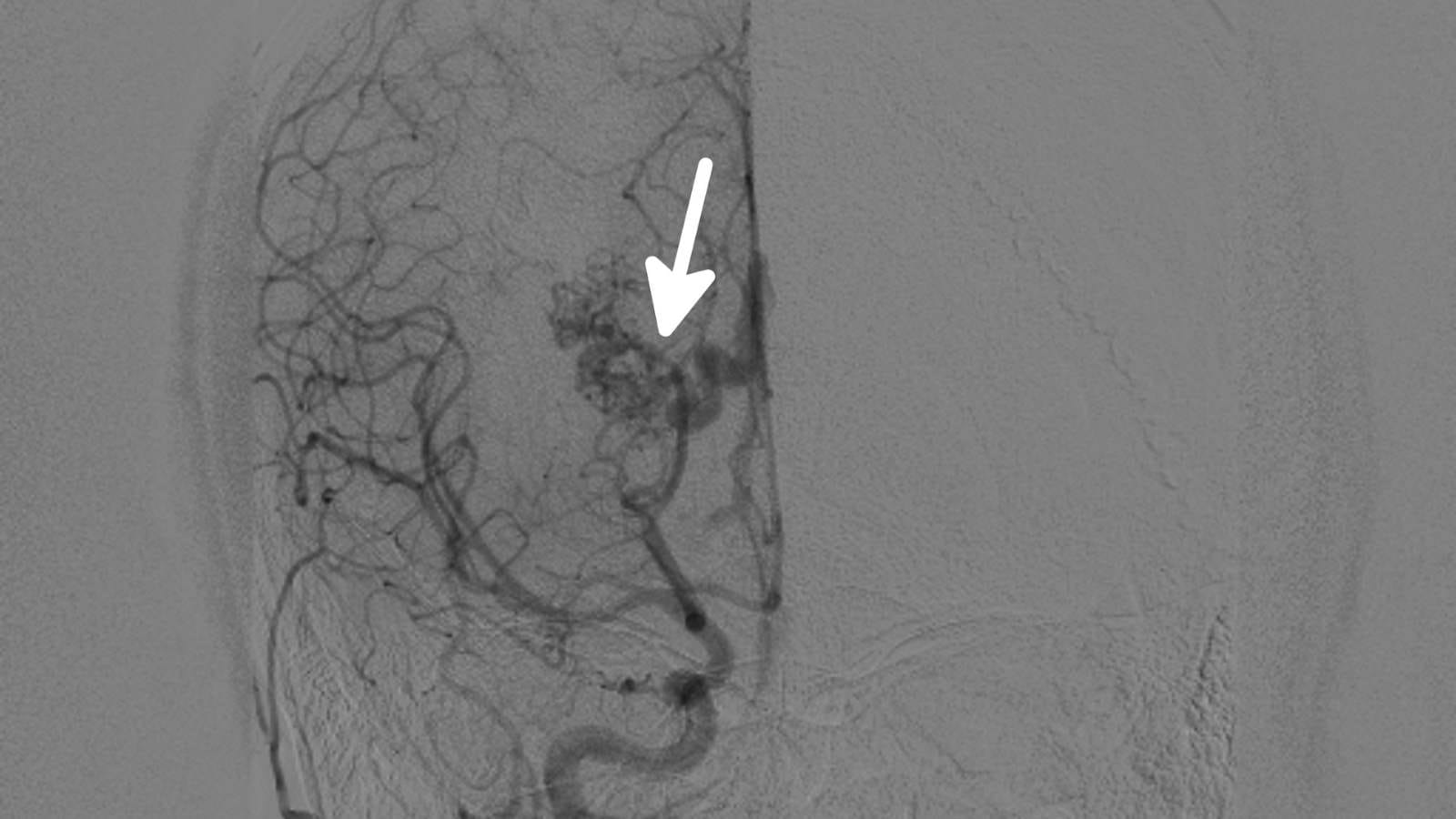
Imaging Guidance: Ultrasound, CT, or MRI is used to guide the needle or catheter to the exact target.
Reaching the Target: Thin needles are advanced to the tumor.
Therapy Delivery: Heat (RFA), cold (cryoablation), chemo and particles (TACE), or radioactive particles (TARE) are applied as indicated.
Control Imaging: After treatment, additional imaging checks the success of the procedure.
Recovery
- Most patients can get up soon after, with only mild pain or fatigue.
- Observation in the hospital usually lasts less than 24 hours; some patients may go home the same day.
- Follow-up imaging is done within weeks to assess the effectiveness.
What Are the Side Effects or Risks?
As with any medical procedure, interventional treatments carry some risks or side effects—but these are typically less severe than with major surgery.
Common examples:
Fever and Pain: Mild to moderate pain or fever may occur after ablation.
Bleeding or Infection: Rare risks at the site of vascular entry.
Procedure Complications: Tumors near major vessels, bile ducts, or nerves may have increased complication risk.
Interventional radiologists use detailed planning and advanced imaging to minimize these risks.
How Do Interventional Treatments Relate to Other Cancer Therapies?
These procedures can be used alone or as part of a multidisciplinary approach:
Before or After Surgery: To shrink tumors or ensure clear margins.
With Chemotherapy or Radiation: To protect organ function or improve tumor control.
With Immunotherapy: Tumor destruction may release antigens that boost immune response, potentially supporting immunotherapy.
Every cancer and patient is unique, so the choice or combination of treatments is individualized.
How Should We Understand the Advantages of Interventional Methods?
These methods are like solo instruments skillfully played within a large musical orchestra. Surgery and chemotherapy are broad-spectrum therapies, but interventional radiology offers more targeted effects. When applied precisely and at the right moment, these methods can enhance the overall outcome—either alone or combined with other treatments.
What Are the Disadvantages or Limitations of These Treatments?
Like all treatments, interventional procedures have limitations:
Tumor Size and Location: Very large or widespread tumors may be less suitable; extra caution is needed near vital vessels or nerves.
Recurrence Risk: Even after complete tumor destruction, recurrence can occur—regular follow-up is essential.
Specialized Equipment and Expertise: These treatments require advanced technology and experienced teams—not always available everywhere.
All pros and cons are discussed in detail with the patient before deciding on the best approach.
Who Is Suitable and Who Is Not?
Not every patient is a candidate for these treatments. They are often preferred for:
- Tumors below a certain size
- Single or limited tumor sites
- Patients who can tolerate anesthesia
- Preserved organ function
- Limited and controllable metastases
If tumors are too widespread, or the patient has severe organ dysfunction or high procedural risk, other options may be considered.
Why Is Follow-Up Important After Treatment?
Even after successful treatment, regular monitoring is essential due to cancer’s unpredictable nature. Just as you revisit a cleared field to check for regrowth, regular follow-ups help detect recurrence or new lesions early.
Follow-up typically involves:
- Periodic imaging (CT, MRI, PET-CT, etc.)
- Blood tests for tumor markers
- Clinical exams and specialist consultations as needed
Early intervention can be planned if recurrence is detected.
What Is the Future of Interventional Treatments?
With rapid advances in medical technology, interventional oncology is constantly evolving. New ablation techniques, advanced imaging, and novel materials are increasing the precision of targeted treatments. Combining these methods with immunotherapy or gene-targeted drugs is expected to bring even more personalized treatment approaches.
For example, research on nanoparticles that highlight cancer cells is ongoing—making it possible to precisely locate and destroy target tissue.
FAQ
**Are these procedures painful?** Generally, local anesthesia or light sedation is used, so patients feel little pain. Any post-procedural pain can usually be managed with painkillers.
Is a single session enough, or will repeat treatments be needed?
It depends on tumor type, size, and response. Small tumors may need only one session; others may require repeat procedures.
Do these treatments cure cancer completely?
The goal is usually complete tumor destruction, but every patient and tumor is unique. Cure rates are higher for early-stage, small tumors; in advanced cases, the goal may be to prolong life and improve quality.
When can I return to daily life after the procedure?
Most patients resume daily activities within the first week after the procedure, though this varies based on the patient’s general condition and procedure scope.

Girişimsel Radyoloji ve Nöroradyoloji Uzmanı Prof. Dr. Özgür Kılıçkesmez, 1997 yılında Cerrahpaşa Tıp Fakültesi’nden mezun oldu. Uzmanlık eğitimini İstanbul Eğitim ve Araştırma Hastanesi’nde tamamladı. Londra’da girişimsel radyoloji ve onkoloji alanında eğitim aldı. İstanbul Çam ve Sakura Şehir Hastanesi’nde girişimsel radyoloji bölümünü kurdu ve 2020 yılında profesör oldu. Çok sayıda uluslararası ödül ve sertifikaya sahip olan Kılıçkesmez’in 150’den fazla bilimsel yayını bulunmakta ve 1500’den fazla atıf almıştır. Halen Medicana Ataköy Hastanesi’nde görev yapmaktadır.




Vaka Örnekleri